Key Takeaways
1. Advanced ECG Technology: The KardiaMobile 6L Max uses a 6-lead ECG system to provide more detailed heart health information compared to standard smartwatches.
2. Early Heart Issue Detection: The device can identify various heart irregularities, reducing the need for expensive and time-consuming clinic visits for a full 12-lead ECG.
3. KardiaCare Subscription: Users must subscribe to KardiaCare for continuous ECG analysis and access to certified cardiologists for early detection of heart problems.
4. Quick and Convenient Use: The device connects via Bluetooth and can produce an ECG reading in just 30 seconds, with data sent to KardiaCare for evaluation through a compatible app.
5. Pricing and Options: The KardiaMobile 6L Max is priced at $169 and will be available on Amazon, with additional portable variations offered.
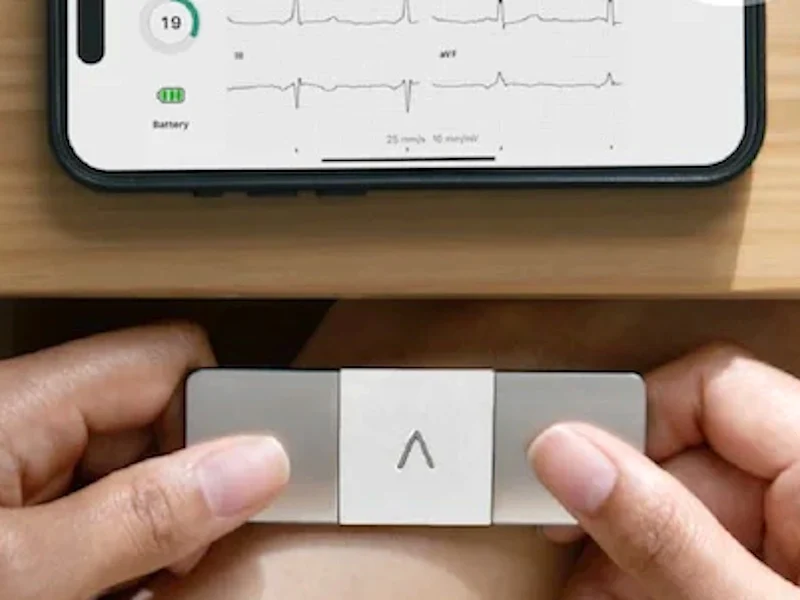
AliveCor has introduced its new KardiaMobile 6L Max, a portable ECG (electrocardiogram) device for smartphones, aimed at identifying heart issues early.
Enhanced Data Capture
The Max offers more valuable information than standard smartwatches by utilizing a straightforward 6-lead ECG system. This gadget can identify various heart irregularities, such as atrial fibrillation (AFib), bradycardia, tachycardia, and sinus rhythm with PVCs, SVE, and Wide QRS. This innovation can lessen the need for frequent visits to the clinic for a full 12-lead ECG, which often requires a lot of time and can be expensive.
Subscription Service for Continuous Monitoring
To benefit from this device, users must sign up for the KardiaCare subscription, which provides continuous ECG analysis. This includes assessments from certified cardiologists when needed, which can be crucial for spotting heart problems early on.
The KardiaMobile 6L Max connects via Bluetooth and can produce an ECG reading in just 30 seconds. The corresponding app can then transmit this data to KardiaCare for further evaluation. The app is compatible with smartphones running Apple iOS 10.3.3 or newer and Android OS 6.0 or above. The device’s dimensions are 9.0 x 3.0 x 0.72 cm (3.5 x 1.2 x 0.28 in.), and it operates on a replaceable lithium coin battery that lasts for a whole year.
Pricing and Availability
The KardiaMobile 6L Max is priced at $169 and will be available shortly on the AliveCor store on Amazon. There are also other variations, including a credit card-sized KardiaMobile for easier portability.
For those interested in understanding how modern diets have led to preventable deaths, especially with heart disease being the top cause of death in the U.S., a relevant book is available on Amazon.
Source:
Link